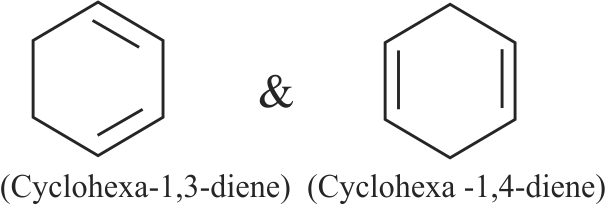
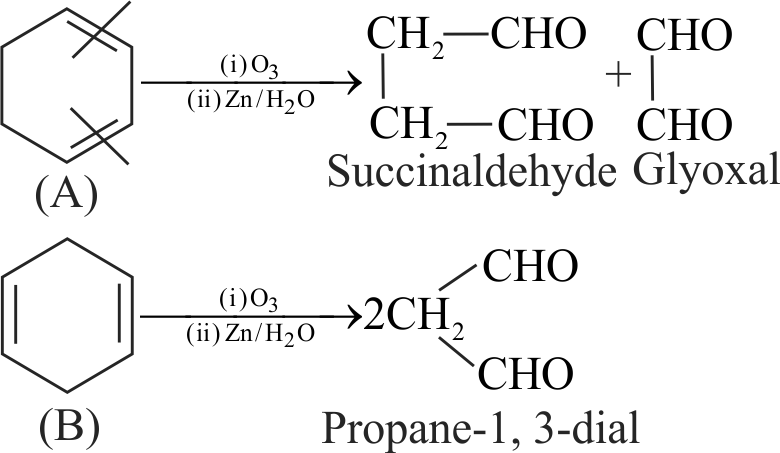
318099 Two cyclic dienes \({\text{A}}\) and \({\text{B}}\) have molecular formula, \(\mathrm{C}_{6} \mathrm{H}_{8}\). On reductive ozonolysis, A gives succinaldehyde and glyoxal while \({\text{B}}\) gives propane-1, 3-dial. A and B on hydrogenation produce only cyclohexane. The total sum of the positions of the double bonds in compounds \(\mathrm{A}\) and \(\mathrm{B}\) is
318099 Two cyclic dienes \({\text{A}}\) and \({\text{B}}\) have molecular formula, \(\mathrm{C}_{6} \mathrm{H}_{8}\). On reductive ozonolysis, A gives succinaldehyde and glyoxal while \({\text{B}}\) gives propane-1, 3-dial. A and B on hydrogenation produce only cyclohexane. The total sum of the positions of the double bonds in compounds \(\mathrm{A}\) and \(\mathrm{B}\) is
318099 Two cyclic dienes \({\text{A}}\) and \({\text{B}}\) have molecular formula, \(\mathrm{C}_{6} \mathrm{H}_{8}\). On reductive ozonolysis, A gives succinaldehyde and glyoxal while \({\text{B}}\) gives propane-1, 3-dial. A and B on hydrogenation produce only cyclohexane. The total sum of the positions of the double bonds in compounds \(\mathrm{A}\) and \(\mathrm{B}\) is
318099 Two cyclic dienes \({\text{A}}\) and \({\text{B}}\) have molecular formula, \(\mathrm{C}_{6} \mathrm{H}_{8}\). On reductive ozonolysis, A gives succinaldehyde and glyoxal while \({\text{B}}\) gives propane-1, 3-dial. A and B on hydrogenation produce only cyclohexane. The total sum of the positions of the double bonds in compounds \(\mathrm{A}\) and \(\mathrm{B}\) is


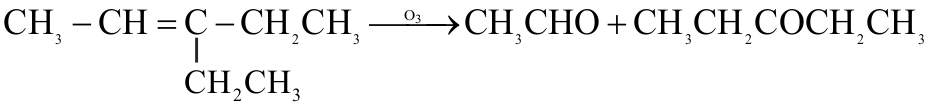
1.jpg)
.jpg)