317861
Which of the following will give three monobromo derivatives?
(A) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}\)
(B) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
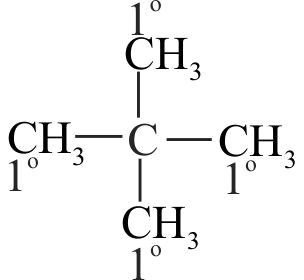
(C) \(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{C}\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CH}_{3}\)
(D) \(\mathrm{CH}_{3} \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
317863
Which of the following statements are correct?
(I) The rate of reactivity of alkanes with halogens is \(\mathrm{F}_{2}>\mathrm{Cl}_{2}>\mathrm{Br}_{2}>\mathrm{I}_{2}\).
(II) Rate of replacement of hydrogens of alkanes is \(3^{\circ}>2^{\circ}>1^{\circ}\)
(III)Fluorination of alkanes is a very slow process.
(IV) Iodination of alkanes is too violent to be controlled.
317861
Which of the following will give three monobromo derivatives?
(A) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}\)
(B) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
(C) \(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{C}\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CH}_{3}\)
(D) \(\mathrm{CH}_{3} \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
317863
Which of the following statements are correct?
(I) The rate of reactivity of alkanes with halogens is \(\mathrm{F}_{2}>\mathrm{Cl}_{2}>\mathrm{Br}_{2}>\mathrm{I}_{2}\).
(II) Rate of replacement of hydrogens of alkanes is \(3^{\circ}>2^{\circ}>1^{\circ}\)
(III)Fluorination of alkanes is a very slow process.
(IV) Iodination of alkanes is too violent to be controlled.
317861
Which of the following will give three monobromo derivatives?
(A) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}\)
(B) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
(C) \(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{C}\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CH}_{3}\)
(D) \(\mathrm{CH}_{3} \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
317863
Which of the following statements are correct?
(I) The rate of reactivity of alkanes with halogens is \(\mathrm{F}_{2}>\mathrm{Cl}_{2}>\mathrm{Br}_{2}>\mathrm{I}_{2}\).
(II) Rate of replacement of hydrogens of alkanes is \(3^{\circ}>2^{\circ}>1^{\circ}\)
(III)Fluorination of alkanes is a very slow process.
(IV) Iodination of alkanes is too violent to be controlled.
317861
Which of the following will give three monobromo derivatives?
(A) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}\)
(B) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
(C) \(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{C}\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CH}_{3}\)
(D) \(\mathrm{CH}_{3} \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}\left(\mathrm{CH}_{3}\right) \mathrm{CH}_{3}\)
317863
Which of the following statements are correct?
(I) The rate of reactivity of alkanes with halogens is \(\mathrm{F}_{2}>\mathrm{Cl}_{2}>\mathrm{Br}_{2}>\mathrm{I}_{2}\).
(II) Rate of replacement of hydrogens of alkanes is \(3^{\circ}>2^{\circ}>1^{\circ}\)
(III)Fluorination of alkanes is a very slow process.
(IV) Iodination of alkanes is too violent to be controlled.
.png)