CHXI11:THE P-BLOCK ELEMENTS
317055
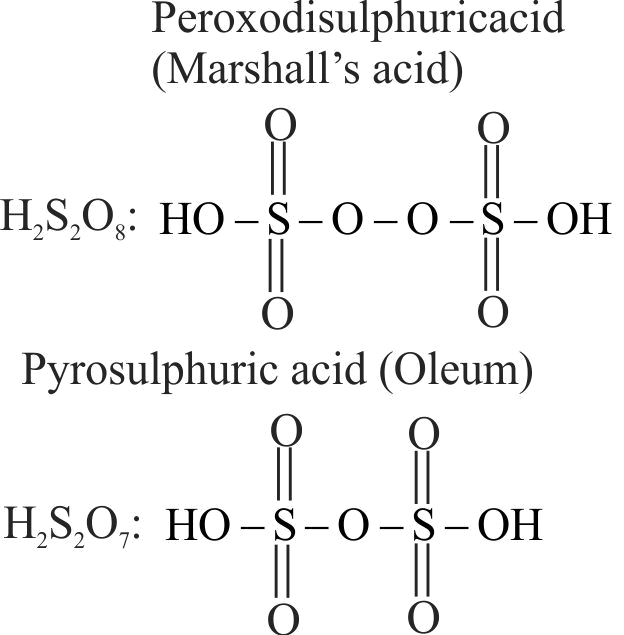
The number of and bonds present in peroxodisulphuric acid and pyrosulphuric acid, respectively are:
1 (2 and 4) and (2 and 4)
2 (4 and 2) and (4 and 2)
3 (4 and 2) and (2 and 4)
4 (2 and 2) and (2 and 2)
Explanation: