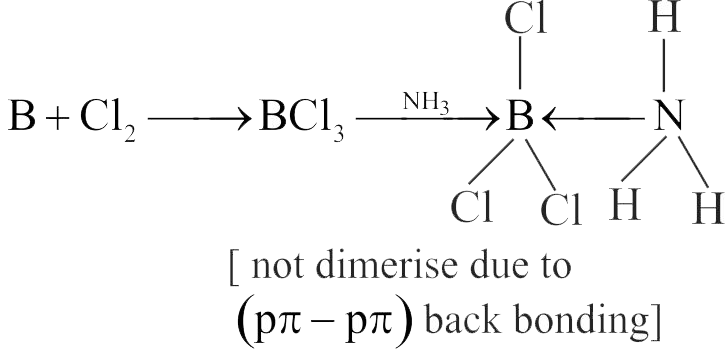
316691 A group 13 element ' \(\mathrm{X}\) ' reacts with chlorine gas to produce a compound \(\mathrm{XCl}_{3}. \mathrm{XCl}_{3}\) is electron deficient and easily reacts with \(\mathrm{NH}_{3}\) to form \(\mathrm{Cl}_{3} \mathrm{X} \leftarrow \mathrm{NH}_{3}\) adduct; however, \(\mathrm{XCl}_{3}\) does not dimerize. \(\mathrm{X}\) is:
316692
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Group 13 trivalent halides get easily hydrolyzed by water due to their covalent nature.
Statement B :
\({\mathrm{\mathrm{\mathrm{AlCl}_{3}}}}\) upon hydrolysis in acidified aqueous solution forms octahedral \({\mathrm{\mathrm{\left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}}}}\) ion.
316691 A group 13 element ' \(\mathrm{X}\) ' reacts with chlorine gas to produce a compound \(\mathrm{XCl}_{3}. \mathrm{XCl}_{3}\) is electron deficient and easily reacts with \(\mathrm{NH}_{3}\) to form \(\mathrm{Cl}_{3} \mathrm{X} \leftarrow \mathrm{NH}_{3}\) adduct; however, \(\mathrm{XCl}_{3}\) does not dimerize. \(\mathrm{X}\) is:
316692
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Group 13 trivalent halides get easily hydrolyzed by water due to their covalent nature.
Statement B :
\({\mathrm{\mathrm{\mathrm{AlCl}_{3}}}}\) upon hydrolysis in acidified aqueous solution forms octahedral \({\mathrm{\mathrm{\left[\mathrm{Al}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+}}}}\) ion.