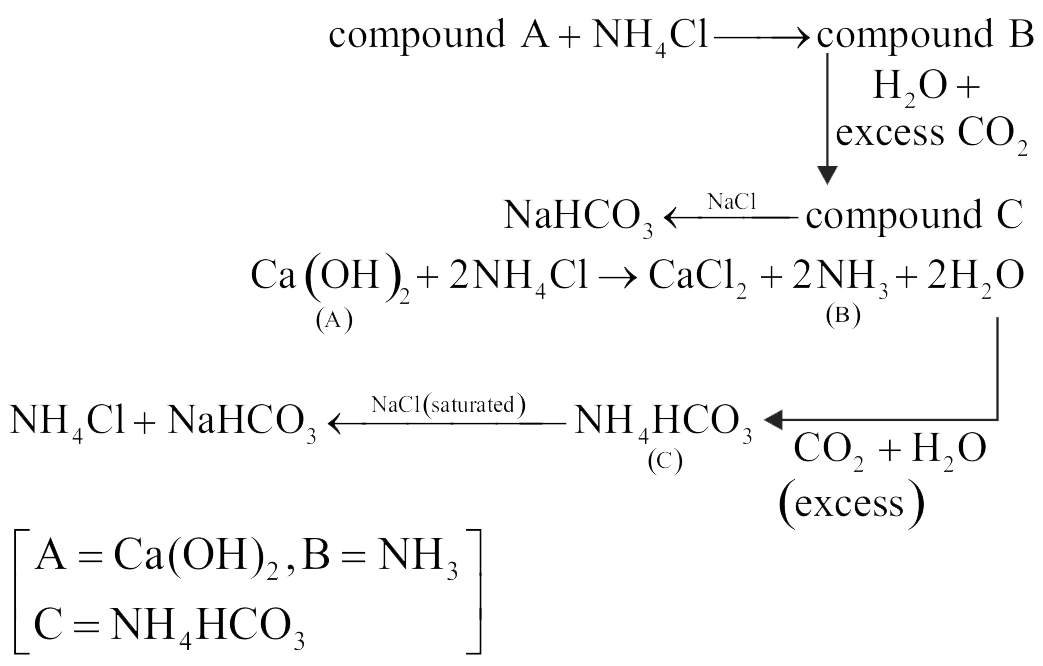
315961 Compound A reacts with \(\mathrm{NH}_{4} \mathrm{Cl}\) and forms a compound B . Compound B reacts \(\mathrm{H}_{2} \mathrm{O}\) with and excess of \(\mathrm{CO}_{2}\) to form compound C which on passing through or reaction with saturated NaCl solution forms sodium hydrogen carbonate. Compound A, B and C, are respectively.
315961 Compound A reacts with \(\mathrm{NH}_{4} \mathrm{Cl}\) and forms a compound B . Compound B reacts \(\mathrm{H}_{2} \mathrm{O}\) with and excess of \(\mathrm{CO}_{2}\) to form compound C which on passing through or reaction with saturated NaCl solution forms sodium hydrogen carbonate. Compound A, B and C, are respectively.
315961 Compound A reacts with \(\mathrm{NH}_{4} \mathrm{Cl}\) and forms a compound B . Compound B reacts \(\mathrm{H}_{2} \mathrm{O}\) with and excess of \(\mathrm{CO}_{2}\) to form compound C which on passing through or reaction with saturated NaCl solution forms sodium hydrogen carbonate. Compound A, B and C, are respectively.
315961 Compound A reacts with \(\mathrm{NH}_{4} \mathrm{Cl}\) and forms a compound B . Compound B reacts \(\mathrm{H}_{2} \mathrm{O}\) with and excess of \(\mathrm{CO}_{2}\) to form compound C which on passing through or reaction with saturated NaCl solution forms sodium hydrogen carbonate. Compound A, B and C, are respectively.