317576
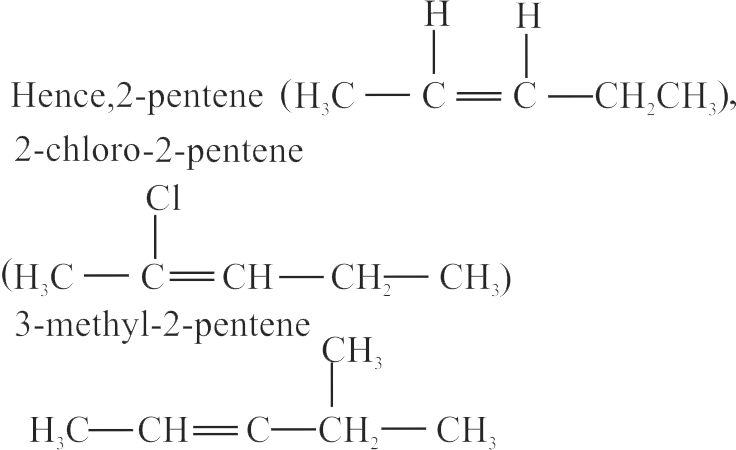
Count total no. of molecules that can show geometrical isomerism.
(a) Isobutene
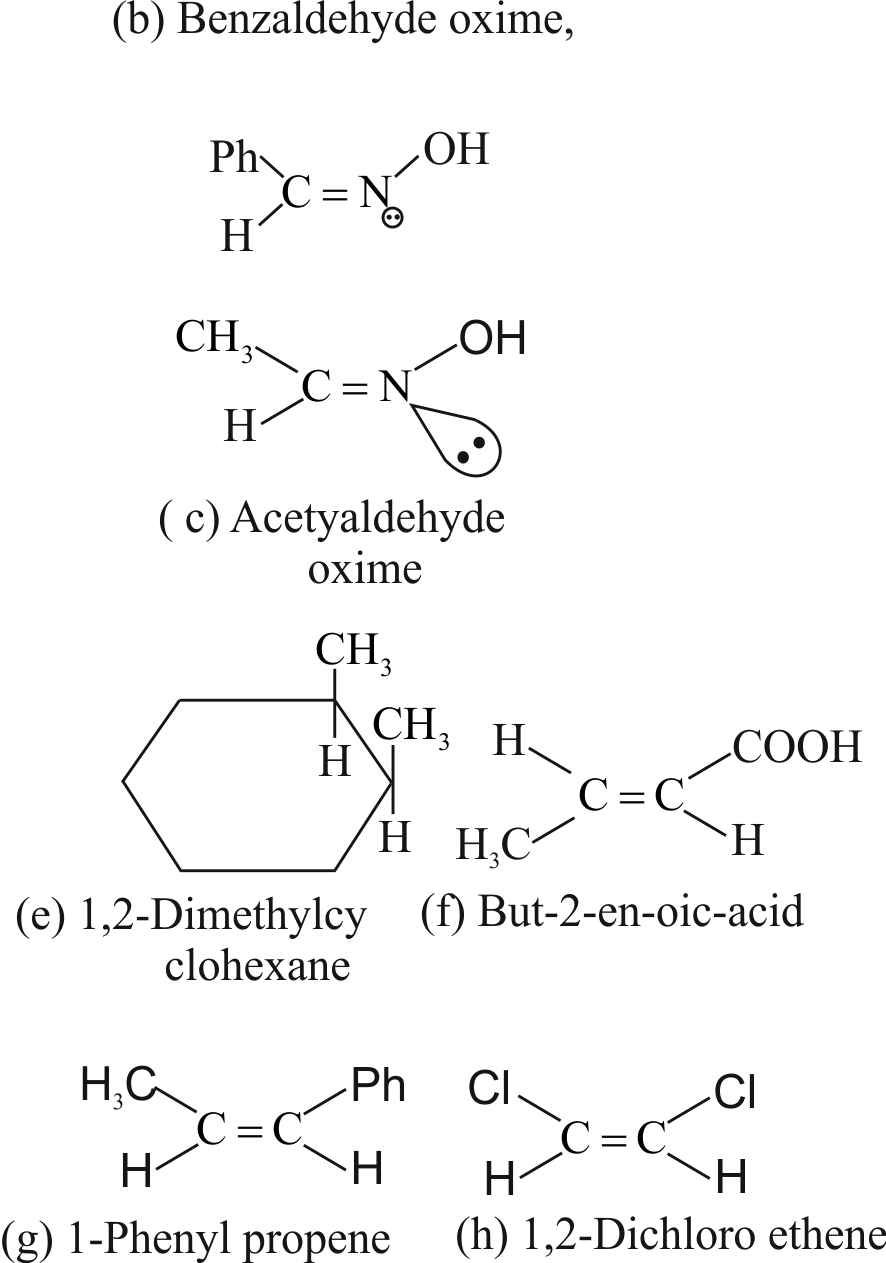
(b) Benzaldehyde-oxime
(c) Acetaldehydeoxime
(d) Cyclohexene
(e) 1,2-Dimethyl cyclohexane
(f) But-2-enoicacid
(g) 1-Phenylpropene
(h) 1,2-Dichloroethene
(i) Pent-2-yne
(j) 1,1-Dichloro cyclohexane
317576
Count total no. of molecules that can show geometrical isomerism.
(a) Isobutene
(b) Benzaldehyde-oxime
(c) Acetaldehydeoxime
(d) Cyclohexene
(e) 1,2-Dimethyl cyclohexane
(f) But-2-enoicacid
(g) 1-Phenylpropene
(h) 1,2-Dichloroethene
(i) Pent-2-yne
(j) 1,1-Dichloro cyclohexane
317576
Count total no. of molecules that can show geometrical isomerism.
(a) Isobutene
(b) Benzaldehyde-oxime
(c) Acetaldehydeoxime
(d) Cyclohexene
(e) 1,2-Dimethyl cyclohexane
(f) But-2-enoicacid
(g) 1-Phenylpropene
(h) 1,2-Dichloroethene
(i) Pent-2-yne
(j) 1,1-Dichloro cyclohexane
317576
Count total no. of molecules that can show geometrical isomerism.
(a) Isobutene
(b) Benzaldehyde-oxime
(c) Acetaldehydeoxime
(d) Cyclohexene
(e) 1,2-Dimethyl cyclohexane
(f) But-2-enoicacid
(g) 1-Phenylpropene
(h) 1,2-Dichloroethene
(i) Pent-2-yne
(j) 1,1-Dichloro cyclohexane