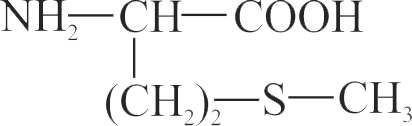
317515 In Carius tube, an organic compound ' \({\text{X}}\) ' is treated with sodium peroxide to form a mineral acid ' Y '. The solution of \(\mathrm{BaCl}_{2}\) is added to ' Y ' to form a precipitate ' Z '. ' Z ' is used for the quantitative estimation of an extra element. ' \({\text{X}}\) ' could be
317515 In Carius tube, an organic compound ' \({\text{X}}\) ' is treated with sodium peroxide to form a mineral acid ' Y '. The solution of \(\mathrm{BaCl}_{2}\) is added to ' Y ' to form a precipitate ' Z '. ' Z ' is used for the quantitative estimation of an extra element. ' \({\text{X}}\) ' could be
317515 In Carius tube, an organic compound ' \({\text{X}}\) ' is treated with sodium peroxide to form a mineral acid ' Y '. The solution of \(\mathrm{BaCl}_{2}\) is added to ' Y ' to form a precipitate ' Z '. ' Z ' is used for the quantitative estimation of an extra element. ' \({\text{X}}\) ' could be
317515 In Carius tube, an organic compound ' \({\text{X}}\) ' is treated with sodium peroxide to form a mineral acid ' Y '. The solution of \(\mathrm{BaCl}_{2}\) is added to ' Y ' to form a precipitate ' Z '. ' Z ' is used for the quantitative estimation of an extra element. ' \({\text{X}}\) ' could be