317461
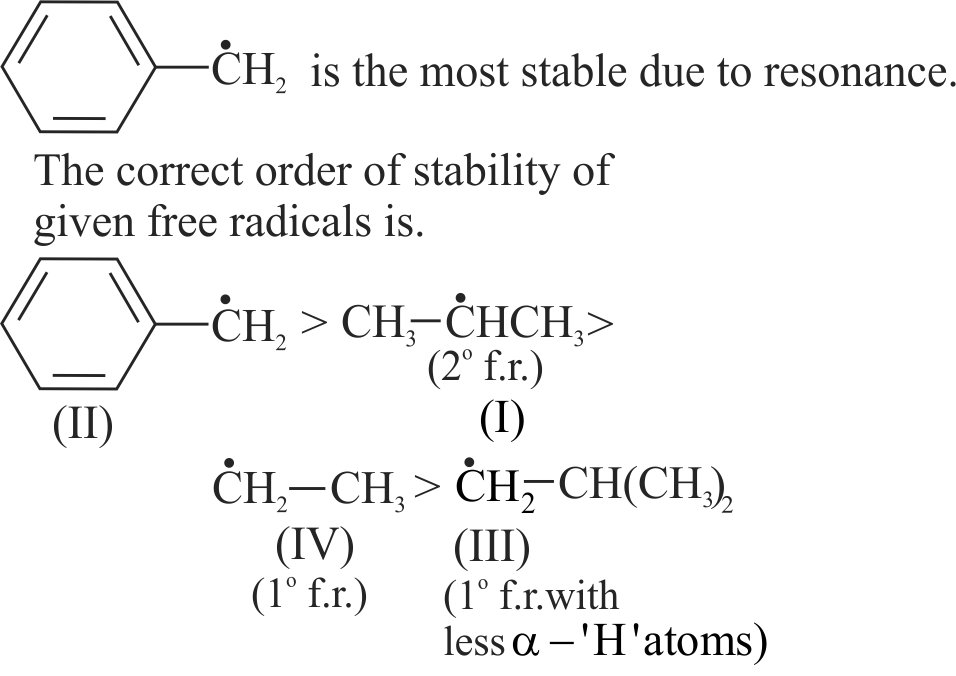
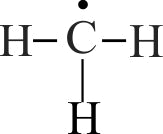
Compare stability of free radicals.
(I) \(\mathrm{CH}_{3}-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)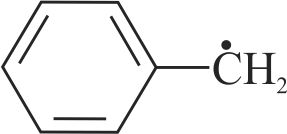
(III) \({ }^{\cdot} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)
(IV) \(\dot{\mathrm{C}} \mathrm{H}_{2}-\mathrm{CH}_{3}\)
317461
Compare stability of free radicals.
(I) \(\mathrm{CH}_{3}-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(III) \({ }^{\cdot} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)
(IV) \(\dot{\mathrm{C}} \mathrm{H}_{2}-\mathrm{CH}_{3}\)
317461
Compare stability of free radicals.
(I) \(\mathrm{CH}_{3}-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(III) \({ }^{\cdot} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)
(IV) \(\dot{\mathrm{C}} \mathrm{H}_{2}-\mathrm{CH}_{3}\)
317461
Compare stability of free radicals.
(I) \(\mathrm{CH}_{3}-\dot{\mathrm{C}} \mathrm{H}-\mathrm{CH}_{3}\)
(III) \({ }^{\cdot} \mathrm{CH}_{2}-\mathrm{CH}\left(\mathrm{CH}_{3}\right)_{2}\)
(IV) \(\dot{\mathrm{C}} \mathrm{H}_{2}-\mathrm{CH}_{3}\)