317454
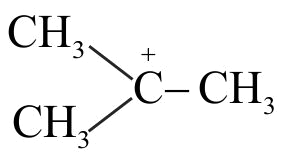
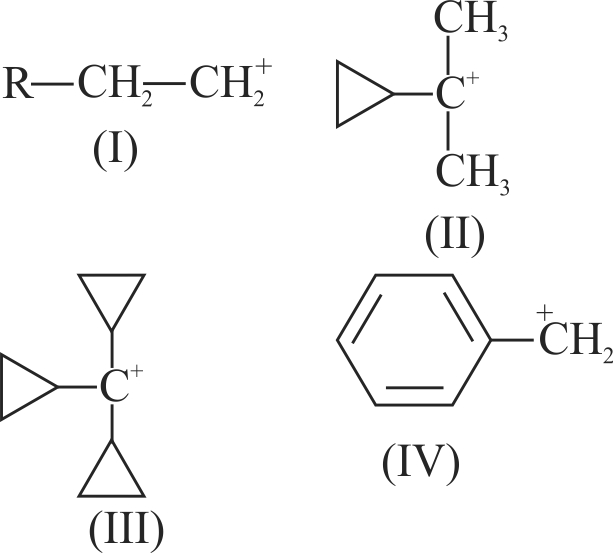
The correct structure of intermediate ' \({\rm{X}}\) ' in the following reaction is
\(\mathrm{CH}_{3} \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_{3}+\mathrm{H}^{+} \stackrel{\text { heat }}{\longrightarrow} \mathrm{X}+\mathrm{H}_{2} \mathrm{O}\)
317454
The correct structure of intermediate ' \({\rm{X}}\) ' in the following reaction is
\(\mathrm{CH}_{3} \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_{3}+\mathrm{H}^{+} \stackrel{\text { heat }}{\longrightarrow} \mathrm{X}+\mathrm{H}_{2} \mathrm{O}\)
317454
The correct structure of intermediate ' \({\rm{X}}\) ' in the following reaction is
\(\mathrm{CH}_{3} \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_{3}+\mathrm{H}^{+} \stackrel{\text { heat }}{\longrightarrow} \mathrm{X}+\mathrm{H}_{2} \mathrm{O}\)
317454
The correct structure of intermediate ' \({\rm{X}}\) ' in the following reaction is
\(\mathrm{CH}_{3} \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_{3}+\mathrm{H}^{+} \stackrel{\text { heat }}{\longrightarrow} \mathrm{X}+\mathrm{H}_{2} \mathrm{O}\)