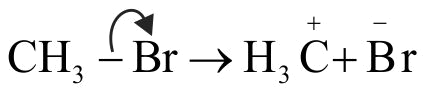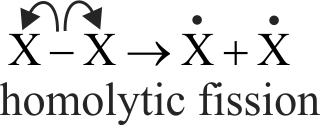317389
Statement A :
A covalent bond may be cleaved either by heterolytic cleavage or by homolytic cleavage.
Statement B :
Heterolytic cleavage of \(\mathrm{CH}_{3} \mathrm{Br}\) will given \(\mathop {\rm{C}}\limits^{\rm{ + }} {{\rm{H}}_{\rm{3}}}{\mkern 1mu} \) and \({\rm{B}}{{\rm{r}}^ - }\)
317390
Assertion :
Heterolytic fission involves the breaking of a covalent bond in such a way that both the electrons of the shared pair are carried away by one of the atoms
Reason :
Heterolytic fission occurs readily in polar covalent bonds.
317389
Statement A :
A covalent bond may be cleaved either by heterolytic cleavage or by homolytic cleavage.
Statement B :
Heterolytic cleavage of \(\mathrm{CH}_{3} \mathrm{Br}\) will given \(\mathop {\rm{C}}\limits^{\rm{ + }} {{\rm{H}}_{\rm{3}}}{\mkern 1mu} \) and \({\rm{B}}{{\rm{r}}^ - }\)
317390
Assertion :
Heterolytic fission involves the breaking of a covalent bond in such a way that both the electrons of the shared pair are carried away by one of the atoms
Reason :
Heterolytic fission occurs readily in polar covalent bonds.
317389
Statement A :
A covalent bond may be cleaved either by heterolytic cleavage or by homolytic cleavage.
Statement B :
Heterolytic cleavage of \(\mathrm{CH}_{3} \mathrm{Br}\) will given \(\mathop {\rm{C}}\limits^{\rm{ + }} {{\rm{H}}_{\rm{3}}}{\mkern 1mu} \) and \({\rm{B}}{{\rm{r}}^ - }\)
317390
Assertion :
Heterolytic fission involves the breaking of a covalent bond in such a way that both the electrons of the shared pair are carried away by one of the atoms
Reason :
Heterolytic fission occurs readily in polar covalent bonds.
317389
Statement A :
A covalent bond may be cleaved either by heterolytic cleavage or by homolytic cleavage.
Statement B :
Heterolytic cleavage of \(\mathrm{CH}_{3} \mathrm{Br}\) will given \(\mathop {\rm{C}}\limits^{\rm{ + }} {{\rm{H}}_{\rm{3}}}{\mkern 1mu} \) and \({\rm{B}}{{\rm{r}}^ - }\)
317390
Assertion :
Heterolytic fission involves the breaking of a covalent bond in such a way that both the electrons of the shared pair are carried away by one of the atoms
Reason :
Heterolytic fission occurs readily in polar covalent bonds.
317389
Statement A :
A covalent bond may be cleaved either by heterolytic cleavage or by homolytic cleavage.
Statement B :
Heterolytic cleavage of \(\mathrm{CH}_{3} \mathrm{Br}\) will given \(\mathop {\rm{C}}\limits^{\rm{ + }} {{\rm{H}}_{\rm{3}}}{\mkern 1mu} \) and \({\rm{B}}{{\rm{r}}^ - }\)
317390
Assertion :
Heterolytic fission involves the breaking of a covalent bond in such a way that both the electrons of the shared pair are carried away by one of the atoms
Reason :
Heterolytic fission occurs readily in polar covalent bonds.