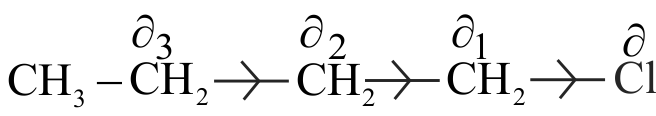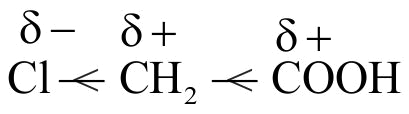317217
Assertion :
Polarisation of one \(\sigma\) bond caused by the polarisation of adjacent \(\sigma\) bond is referred to as the inductive effect.
Reason :
The substituents can be classified as electron withdrawing or electron donating groups relative to hydrogen.
317217
Assertion :
Polarisation of one \(\sigma\) bond caused by the polarisation of adjacent \(\sigma\) bond is referred to as the inductive effect.
Reason :
The substituents can be classified as electron withdrawing or electron donating groups relative to hydrogen.
317217
Assertion :
Polarisation of one \(\sigma\) bond caused by the polarisation of adjacent \(\sigma\) bond is referred to as the inductive effect.
Reason :
The substituents can be classified as electron withdrawing or electron donating groups relative to hydrogen.
317217
Assertion :
Polarisation of one \(\sigma\) bond caused by the polarisation of adjacent \(\sigma\) bond is referred to as the inductive effect.
Reason :
The substituents can be classified as electron withdrawing or electron donating groups relative to hydrogen.
317217
Assertion :
Polarisation of one \(\sigma\) bond caused by the polarisation of adjacent \(\sigma\) bond is referred to as the inductive effect.
Reason :
The substituents can be classified as electron withdrawing or electron donating groups relative to hydrogen.