317268
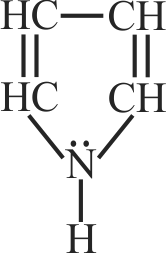
Consider the following statements.
I. The resonance structure have different positions of nuclei.
II. Resonance structures have the same number of unpaired electrons.
III. Among the resonance structures, the one which has more number of covalent bonds, all the atoms with octet of electrons and more dispersal of charge is more stable than others.
Read the given statements and choose the incorrect option.
317268
Consider the following statements.
I. The resonance structure have different positions of nuclei.
II. Resonance structures have the same number of unpaired electrons.
III. Among the resonance structures, the one which has more number of covalent bonds, all the atoms with octet of electrons and more dispersal of charge is more stable than others.
Read the given statements and choose the incorrect option.
317268
Consider the following statements.
I. The resonance structure have different positions of nuclei.
II. Resonance structures have the same number of unpaired electrons.
III. Among the resonance structures, the one which has more number of covalent bonds, all the atoms with octet of electrons and more dispersal of charge is more stable than others.
Read the given statements and choose the incorrect option.
317268
Consider the following statements.
I. The resonance structure have different positions of nuclei.
II. Resonance structures have the same number of unpaired electrons.
III. Among the resonance structures, the one which has more number of covalent bonds, all the atoms with octet of electrons and more dispersal of charge is more stable than others.
Read the given statements and choose the incorrect option.