315593
Which of the following set is having correct statements?
(A) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) is a weak base
(B) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) oxidizes \(\mathrm{\mathrm{Fe}}\) (II) to \(\mathrm{\mathrm{Fe}}\) (III)
(C) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) can be obtained by the electrolysis of conc. \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\)
(D) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) reduces \(\mathrm{\mathrm{Mn}(\mathrm{VII})}\) to \(\mathrm{\mathrm{Mn}(\mathrm{II})}\)
315594
Consider the reactions
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + 2HI}} \to {{\text{I}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({\text{HOCl + }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}} \to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}{\text{ + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(ii)}}\)
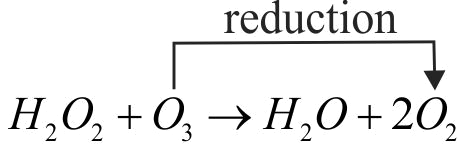
Which of the following statement is correct about \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) with reference to these reactions? Hydrogen peroxide acts as
315593
Which of the following set is having correct statements?
(A) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) is a weak base
(B) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) oxidizes \(\mathrm{\mathrm{Fe}}\) (II) to \(\mathrm{\mathrm{Fe}}\) (III)
(C) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) can be obtained by the electrolysis of conc. \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\)
(D) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) reduces \(\mathrm{\mathrm{Mn}(\mathrm{VII})}\) to \(\mathrm{\mathrm{Mn}(\mathrm{II})}\)
315594
Consider the reactions
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + 2HI}} \to {{\text{I}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({\text{HOCl + }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}} \to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}{\text{ + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(ii)}}\)
Which of the following statement is correct about \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) with reference to these reactions? Hydrogen peroxide acts as
315593
Which of the following set is having correct statements?
(A) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) is a weak base
(B) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) oxidizes \(\mathrm{\mathrm{Fe}}\) (II) to \(\mathrm{\mathrm{Fe}}\) (III)
(C) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) can be obtained by the electrolysis of conc. \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\)
(D) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) reduces \(\mathrm{\mathrm{Mn}(\mathrm{VII})}\) to \(\mathrm{\mathrm{Mn}(\mathrm{II})}\)
315594
Consider the reactions
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + 2HI}} \to {{\text{I}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({\text{HOCl + }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}} \to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}{\text{ + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(ii)}}\)
Which of the following statement is correct about \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) with reference to these reactions? Hydrogen peroxide acts as
315593
Which of the following set is having correct statements?
(A) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) is a weak base
(B) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) oxidizes \(\mathrm{\mathrm{Fe}}\) (II) to \(\mathrm{\mathrm{Fe}}\) (III)
(C) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) can be obtained by the electrolysis of conc. \(\mathrm{\mathrm{H}_{2} \mathrm{SO}_{4}}\)
(D) \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) reduces \(\mathrm{\mathrm{Mn}(\mathrm{VII})}\) to \(\mathrm{\mathrm{Mn}(\mathrm{II})}\)
315594
Consider the reactions
\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{ + 2HI}} \to {{\text{I}}_{\text{2}}}{\text{ + 2}}{{\text{H}}_{\text{2}}}{\text{O}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} \,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(i)}}\)
\({\text{HOCl + }}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}} \to {{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}{\text{ + C}}{{\text{l}}^{\text{ - }}}{\text{ + }}{{\text{O}}_{\text{2}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(ii)}}\)
Which of the following statement is correct about \(\mathrm{\mathrm{H}_{2} \mathrm{O}_{2}}\) with reference to these reactions? Hydrogen peroxide acts as