315442
Which of the following options are correct for the reaction
\(2\left[\mathrm{Au}(\mathrm{CN})_{2}\right]_{(\mathrm{aq})}^{-}+\mathrm{Zn}_{(\mathrm{s})} \rightarrow\) \(2 \mathrm{Au}_{(\mathrm{s})}+\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]_{(\mathrm{aq})}^{2-}\)
(A) Redox reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Combination reaction
Choose the correct answer from the options given below:
315444
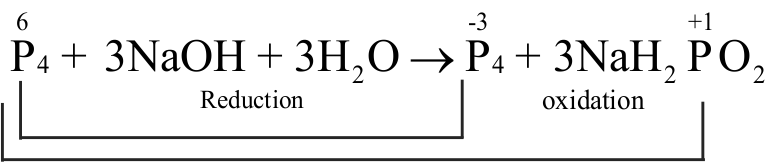
Assertion :
When white \(\mathrm{P}_{4}\) reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of disproportionation reaction.
Reason :
In disproportionation reaction, the same substance may act simultaneously as an oxidising agent and as a reducing agent.
315442
Which of the following options are correct for the reaction
\(2\left[\mathrm{Au}(\mathrm{CN})_{2}\right]_{(\mathrm{aq})}^{-}+\mathrm{Zn}_{(\mathrm{s})} \rightarrow\) \(2 \mathrm{Au}_{(\mathrm{s})}+\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]_{(\mathrm{aq})}^{2-}\)
(A) Redox reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Combination reaction
Choose the correct answer from the options given below:
315444
Assertion :
When white \(\mathrm{P}_{4}\) reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of disproportionation reaction.
Reason :
In disproportionation reaction, the same substance may act simultaneously as an oxidising agent and as a reducing agent.
315442
Which of the following options are correct for the reaction
\(2\left[\mathrm{Au}(\mathrm{CN})_{2}\right]_{(\mathrm{aq})}^{-}+\mathrm{Zn}_{(\mathrm{s})} \rightarrow\) \(2 \mathrm{Au}_{(\mathrm{s})}+\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]_{(\mathrm{aq})}^{2-}\)
(A) Redox reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Combination reaction
Choose the correct answer from the options given below:
315444
Assertion :
When white \(\mathrm{P}_{4}\) reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of disproportionation reaction.
Reason :
In disproportionation reaction, the same substance may act simultaneously as an oxidising agent and as a reducing agent.
315442
Which of the following options are correct for the reaction
\(2\left[\mathrm{Au}(\mathrm{CN})_{2}\right]_{(\mathrm{aq})}^{-}+\mathrm{Zn}_{(\mathrm{s})} \rightarrow\) \(2 \mathrm{Au}_{(\mathrm{s})}+\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]_{(\mathrm{aq})}^{2-}\)
(A) Redox reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Combination reaction
Choose the correct answer from the options given below:
315444
Assertion :
When white \(\mathrm{P}_{4}\) reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of disproportionation reaction.
Reason :
In disproportionation reaction, the same substance may act simultaneously as an oxidising agent and as a reducing agent.
315442
Which of the following options are correct for the reaction
\(2\left[\mathrm{Au}(\mathrm{CN})_{2}\right]_{(\mathrm{aq})}^{-}+\mathrm{Zn}_{(\mathrm{s})} \rightarrow\) \(2 \mathrm{Au}_{(\mathrm{s})}+\left[\mathrm{Zn}(\mathrm{CN})_{4}\right]_{(\mathrm{aq})}^{2-}\)
(A) Redox reaction
(B) Displacement reaction
(C) Decomposition reaction
(D) Combination reaction
Choose the correct answer from the options given below:
315444
Assertion :
When white \(\mathrm{P}_{4}\) reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of disproportionation reaction.
Reason :
In disproportionation reaction, the same substance may act simultaneously as an oxidising agent and as a reducing agent.