315243
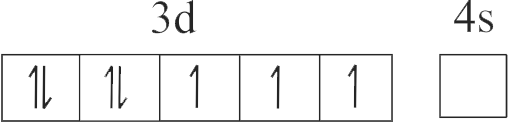
The number of species from the following having +2 oxidation state of metal ion is ____ .
\({\mathrm{\mathrm{FeO}, \mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{CuI}, \mathrm{CuO}, \mathrm{SnCl}_{2}, \mathrm{SnCl}_{4}, \mathrm{Hg}_{2} \mathrm{Cl}_{2}}}\), \({\mathrm{\mathrm{HgCl}_{2}}}\)
315243
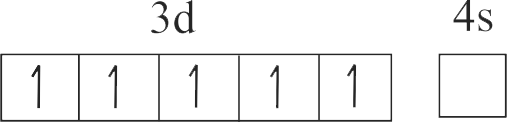
The number of species from the following having +2 oxidation state of metal ion is ____ .
\({\mathrm{\mathrm{FeO}, \mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{CuI}, \mathrm{CuO}, \mathrm{SnCl}_{2}, \mathrm{SnCl}_{4}, \mathrm{Hg}_{2} \mathrm{Cl}_{2}}}\), \({\mathrm{\mathrm{HgCl}_{2}}}\)
315243
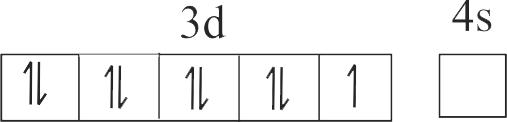
The number of species from the following having +2 oxidation state of metal ion is ____ .
\({\mathrm{\mathrm{FeO}, \mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{CuI}, \mathrm{CuO}, \mathrm{SnCl}_{2}, \mathrm{SnCl}_{4}, \mathrm{Hg}_{2} \mathrm{Cl}_{2}}}\), \({\mathrm{\mathrm{HgCl}_{2}}}\)
315243
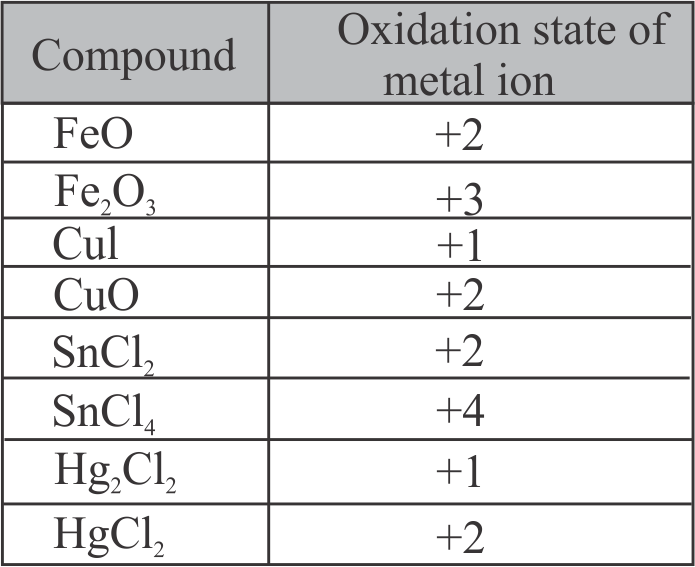
The number of species from the following having +2 oxidation state of metal ion is ____ .
\({\mathrm{\mathrm{FeO}, \mathrm{Fe}_{2} \mathrm{O}_{3}, \mathrm{CuI}, \mathrm{CuO}, \mathrm{SnCl}_{2}, \mathrm{SnCl}_{4}, \mathrm{Hg}_{2} \mathrm{Cl}_{2}}}\), \({\mathrm{\mathrm{HgCl}_{2}}}\)