313952
Consider the following statements :
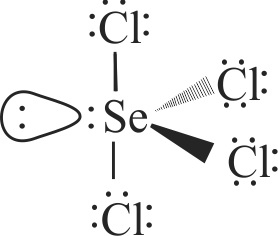
(I) \({\rm{N}}{{\rm{F}}_3}\) molecule has a trigonal planar structure.
(II) Bond length of \({{\rm{N}}_2}\) is shorter than \({{\rm{O}}_2}\).
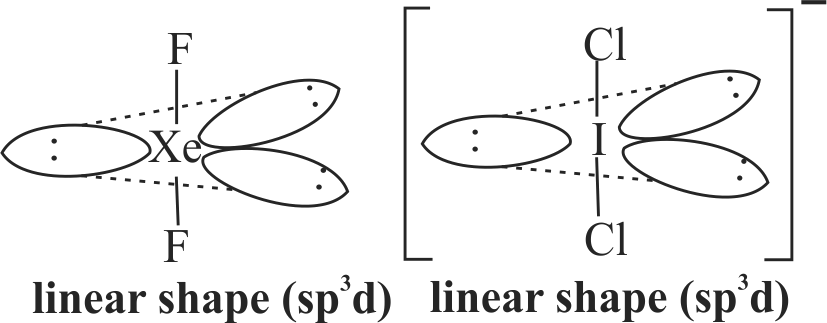
(III) Isoelectronic molecules or ions have identical bond order.
(IV) Dipole moment of \({{\rm{H}}_2}\;{\rm{S}}\) is higher than that of water molecule.
Choose the correct answer from the options given below:
313952
Consider the following statements :
(I) \({\rm{N}}{{\rm{F}}_3}\) molecule has a trigonal planar structure.
(II) Bond length of \({{\rm{N}}_2}\) is shorter than \({{\rm{O}}_2}\).
(III) Isoelectronic molecules or ions have identical bond order.
(IV) Dipole moment of \({{\rm{H}}_2}\;{\rm{S}}\) is higher than that of water molecule.
Choose the correct answer from the options given below:
313952
Consider the following statements :
(I) \({\rm{N}}{{\rm{F}}_3}\) molecule has a trigonal planar structure.
(II) Bond length of \({{\rm{N}}_2}\) is shorter than \({{\rm{O}}_2}\).
(III) Isoelectronic molecules or ions have identical bond order.
(IV) Dipole moment of \({{\rm{H}}_2}\;{\rm{S}}\) is higher than that of water molecule.
Choose the correct answer from the options given below:
313952
Consider the following statements :
(I) \({\rm{N}}{{\rm{F}}_3}\) molecule has a trigonal planar structure.
(II) Bond length of \({{\rm{N}}_2}\) is shorter than \({{\rm{O}}_2}\).
(III) Isoelectronic molecules or ions have identical bond order.
(IV) Dipole moment of \({{\rm{H}}_2}\;{\rm{S}}\) is higher than that of water molecule.
Choose the correct answer from the options given below: