313941
Read the Statement A and Statement B carefully and mark correct option.
Statement A :
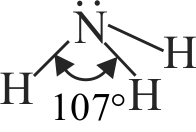
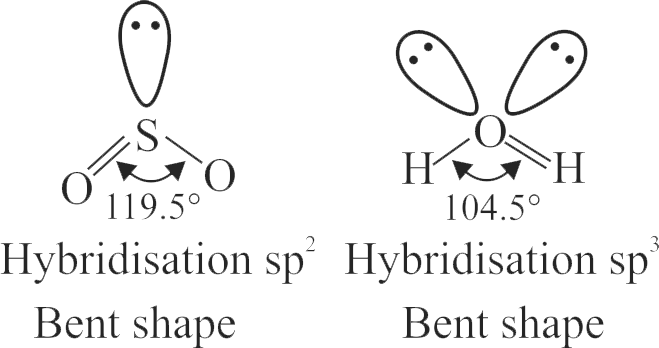
\({\rm{S}}{{\rm{O}}_2}\) and \({{\rm{H}}_2}{\rm{O}}\) both possess V shaped structure.
Statement B :
The bond angle of \({\rm{S}}{{\rm{O}}_2}\) is less than that of \({{\rm{H}}_2}{\rm{O}}\).
313941
Read the Statement A and Statement B carefully and mark correct option.
Statement A :
\({\rm{S}}{{\rm{O}}_2}\) and \({{\rm{H}}_2}{\rm{O}}\) both possess V shaped structure.
Statement B :
The bond angle of \({\rm{S}}{{\rm{O}}_2}\) is less than that of \({{\rm{H}}_2}{\rm{O}}\).
313941
Read the Statement A and Statement B carefully and mark correct option.
Statement A :
\({\rm{S}}{{\rm{O}}_2}\) and \({{\rm{H}}_2}{\rm{O}}\) both possess V shaped structure.
Statement B :
The bond angle of \({\rm{S}}{{\rm{O}}_2}\) is less than that of \({{\rm{H}}_2}{\rm{O}}\).
313941
Read the Statement A and Statement B carefully and mark correct option.
Statement A :
\({\rm{S}}{{\rm{O}}_2}\) and \({{\rm{H}}_2}{\rm{O}}\) both possess V shaped structure.
Statement B :
The bond angle of \({\rm{S}}{{\rm{O}}_2}\) is less than that of \({{\rm{H}}_2}{\rm{O}}\).