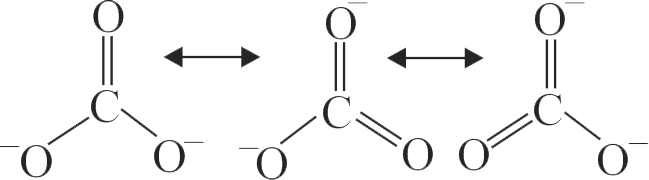
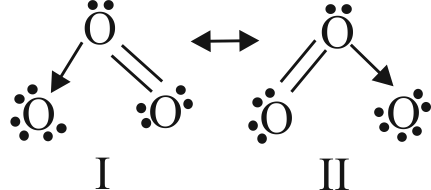
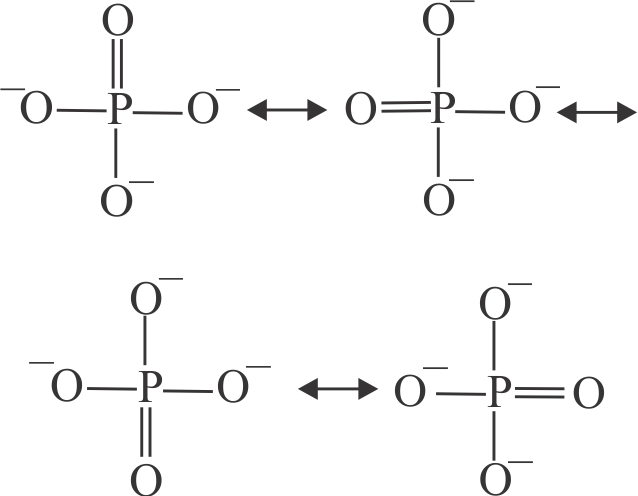
313903 A molecule may be represented by three structures having energies \({{\rm{E}}_{\rm{1}}}{\rm{,}}{{\rm{E}}_{\rm{2}}}\,\,{\rm{and}}\,\,{{\rm{E}}_{\rm{3}}}\) respectively. The energies of these structures follow the order \({{\rm{E}}_{\rm{3}}}{\rm{ < }}{{\rm{E}}_{\rm{2}}}{\rm{ < }}{{\rm{E}}_{\rm{1}}}\) respectively. If the experimental energy of the molecule is \({{\rm{E}}_{\rm{0}}}\), the resonance energy is
313903 A molecule may be represented by three structures having energies \({{\rm{E}}_{\rm{1}}}{\rm{,}}{{\rm{E}}_{\rm{2}}}\,\,{\rm{and}}\,\,{{\rm{E}}_{\rm{3}}}\) respectively. The energies of these structures follow the order \({{\rm{E}}_{\rm{3}}}{\rm{ < }}{{\rm{E}}_{\rm{2}}}{\rm{ < }}{{\rm{E}}_{\rm{1}}}\) respectively. If the experimental energy of the molecule is \({{\rm{E}}_{\rm{0}}}\), the resonance energy is
313903 A molecule may be represented by three structures having energies \({{\rm{E}}_{\rm{1}}}{\rm{,}}{{\rm{E}}_{\rm{2}}}\,\,{\rm{and}}\,\,{{\rm{E}}_{\rm{3}}}\) respectively. The energies of these structures follow the order \({{\rm{E}}_{\rm{3}}}{\rm{ < }}{{\rm{E}}_{\rm{2}}}{\rm{ < }}{{\rm{E}}_{\rm{1}}}\) respectively. If the experimental energy of the molecule is \({{\rm{E}}_{\rm{0}}}\), the resonance energy is
313903 A molecule may be represented by three structures having energies \({{\rm{E}}_{\rm{1}}}{\rm{,}}{{\rm{E}}_{\rm{2}}}\,\,{\rm{and}}\,\,{{\rm{E}}_{\rm{3}}}\) respectively. The energies of these structures follow the order \({{\rm{E}}_{\rm{3}}}{\rm{ < }}{{\rm{E}}_{\rm{2}}}{\rm{ < }}{{\rm{E}}_{\rm{1}}}\) respectively. If the experimental energy of the molecule is \({{\rm{E}}_{\rm{0}}}\), the resonance energy is
313903 A molecule may be represented by three structures having energies \({{\rm{E}}_{\rm{1}}}{\rm{,}}{{\rm{E}}_{\rm{2}}}\,\,{\rm{and}}\,\,{{\rm{E}}_{\rm{3}}}\) respectively. The energies of these structures follow the order \({{\rm{E}}_{\rm{3}}}{\rm{ < }}{{\rm{E}}_{\rm{2}}}{\rm{ < }}{{\rm{E}}_{\rm{1}}}\) respectively. If the experimental energy of the molecule is \({{\rm{E}}_{\rm{0}}}\), the resonance energy is