313853
How many molecules have zero dipole moment?
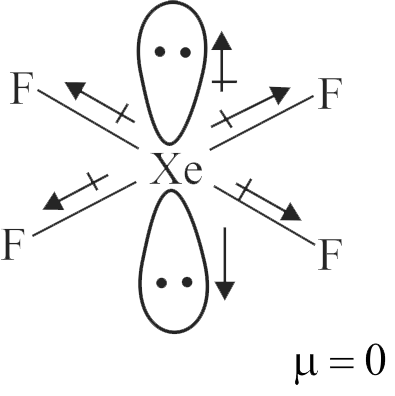
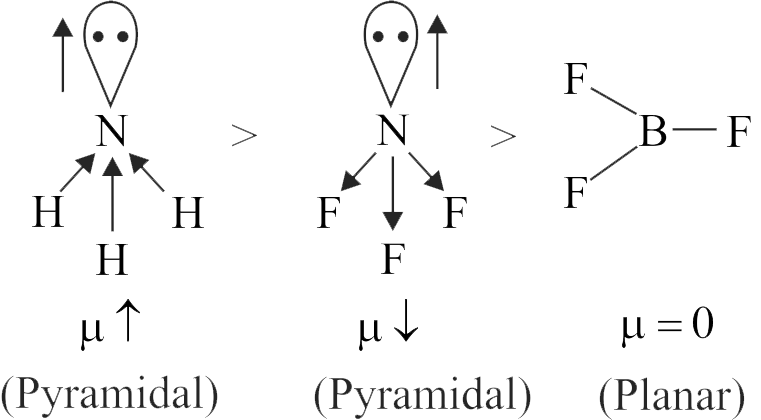
\({\rm{PC}}{{\rm{l}}_{\rm{3}}}{{\rm{F}}_{\rm{2}}}{\rm{,}}\,\,{\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{,}}\,\,{\rm{B}}{{\rm{F}}_{\rm{3}}}{\rm{,}}\,\,{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\)
\({\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{,}}\,\,{\rm{P}}{{\rm{F}}_{\rm{3}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{,}}\,\,{\rm{C}}{{\rm{H}}_{\rm{4}}}{\rm{,}}\,\,{\rm{CC}}{{\rm{l}}_{\rm{4}}}\)
313853
How many molecules have zero dipole moment?
\({\rm{PC}}{{\rm{l}}_{\rm{3}}}{{\rm{F}}_{\rm{2}}}{\rm{,}}\,\,{\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{,}}\,\,{\rm{B}}{{\rm{F}}_{\rm{3}}}{\rm{,}}\,\,{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\)
\({\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{,}}\,\,{\rm{P}}{{\rm{F}}_{\rm{3}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{,}}\,\,{\rm{C}}{{\rm{H}}_{\rm{4}}}{\rm{,}}\,\,{\rm{CC}}{{\rm{l}}_{\rm{4}}}\)
313853
How many molecules have zero dipole moment?
\({\rm{PC}}{{\rm{l}}_{\rm{3}}}{{\rm{F}}_{\rm{2}}}{\rm{,}}\,\,{\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{,}}\,\,{\rm{B}}{{\rm{F}}_{\rm{3}}}{\rm{,}}\,\,{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\)
\({\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{,}}\,\,{\rm{P}}{{\rm{F}}_{\rm{3}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{,}}\,\,{\rm{C}}{{\rm{H}}_{\rm{4}}}{\rm{,}}\,\,{\rm{CC}}{{\rm{l}}_{\rm{4}}}\)
313853
How many molecules have zero dipole moment?
\({\rm{PC}}{{\rm{l}}_{\rm{3}}}{{\rm{F}}_{\rm{2}}}{\rm{,}}\,\,{\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{,}}\,\,{\rm{B}}{{\rm{F}}_{\rm{3}}}{\rm{,}}\,\,{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\)
\({\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{,}}\,\,{\rm{P}}{{\rm{F}}_{\rm{3}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{,}}\,\,{\rm{C}}{{\rm{H}}_{\rm{4}}}{\rm{,}}\,\,{\rm{CC}}{{\rm{l}}_{\rm{4}}}\)
313853
How many molecules have zero dipole moment?
\({\rm{PC}}{{\rm{l}}_{\rm{3}}}{{\rm{F}}_{\rm{2}}}{\rm{,}}\,\,{\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{2}}}{\rm{,}}\,\,{\rm{B}}{{\rm{F}}_{\rm{3}}}{\rm{,}}\,\,{\rm{CHC}}{{\rm{l}}_{\rm{3}}}\)
\({\rm{P}}{\left( {{\rm{C}}{{\rm{H}}_{\rm{3}}}} \right)_{\rm{2}}}{\left( {{\rm{C}}{{\rm{F}}_{\rm{3}}}} \right)_{\rm{3}}}{\rm{,}}\,\,{\rm{P}}{{\rm{F}}_{\rm{3}}}{\rm{C}}{{\rm{l}}_{\rm{2}}}{\rm{,}}\,\,{\rm{C}}{{\rm{H}}_{\rm{4}}}{\rm{,}}\,\,{\rm{CC}}{{\rm{l}}_{\rm{4}}}\)