CHXI04:CHEMICAL BONDING AND MOLECULAR STRUCTURE
313899
Which one of the following molecules is polar?
1 \({\rm{Xe}}{{\rm{F}}_{\rm{4}}}\)
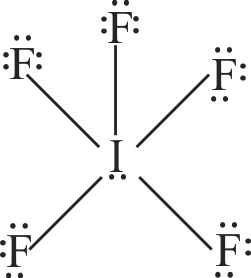
2 \({\rm{I}}{{\rm{F}}_{\rm{5}}}\)
3 \({\rm{Sb}}{{\rm{F}}_{\rm{5}}}\)
4 \({\rm{C}}{{\rm{F}}_{\rm{4}}}\)
Explanation:
The geometry of \({\rm{I}}{{\rm{F}}_{\rm{5}}}\) is square pyramidal with an unsymmetric charge distribution therefore this molecule is polar.