313682
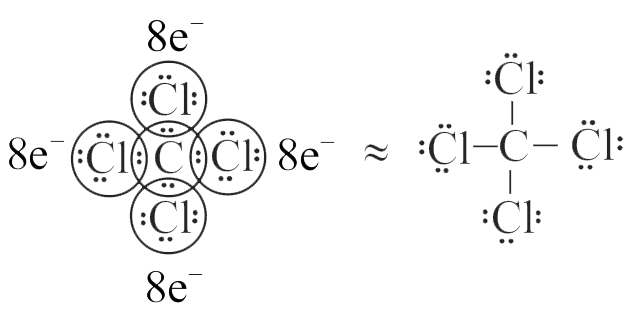
Which of the following conditions are fulfilled by the Lewis dot structure for carbon tetrachloride?
(I) Each covalent bond is formed by sharing of an electron pair between the atoms.
(II) Each combining atom contributes at least one electron to the shared pair.
(III) The combining atoms attain noble gas configurations because of the sharing of electrons.
313682
Which of the following conditions are fulfilled by the Lewis dot structure for carbon tetrachloride?
(I) Each covalent bond is formed by sharing of an electron pair between the atoms.
(II) Each combining atom contributes at least one electron to the shared pair.
(III) The combining atoms attain noble gas configurations because of the sharing of electrons.
313682
Which of the following conditions are fulfilled by the Lewis dot structure for carbon tetrachloride?
(I) Each covalent bond is formed by sharing of an electron pair between the atoms.
(II) Each combining atom contributes at least one electron to the shared pair.
(III) The combining atoms attain noble gas configurations because of the sharing of electrons.