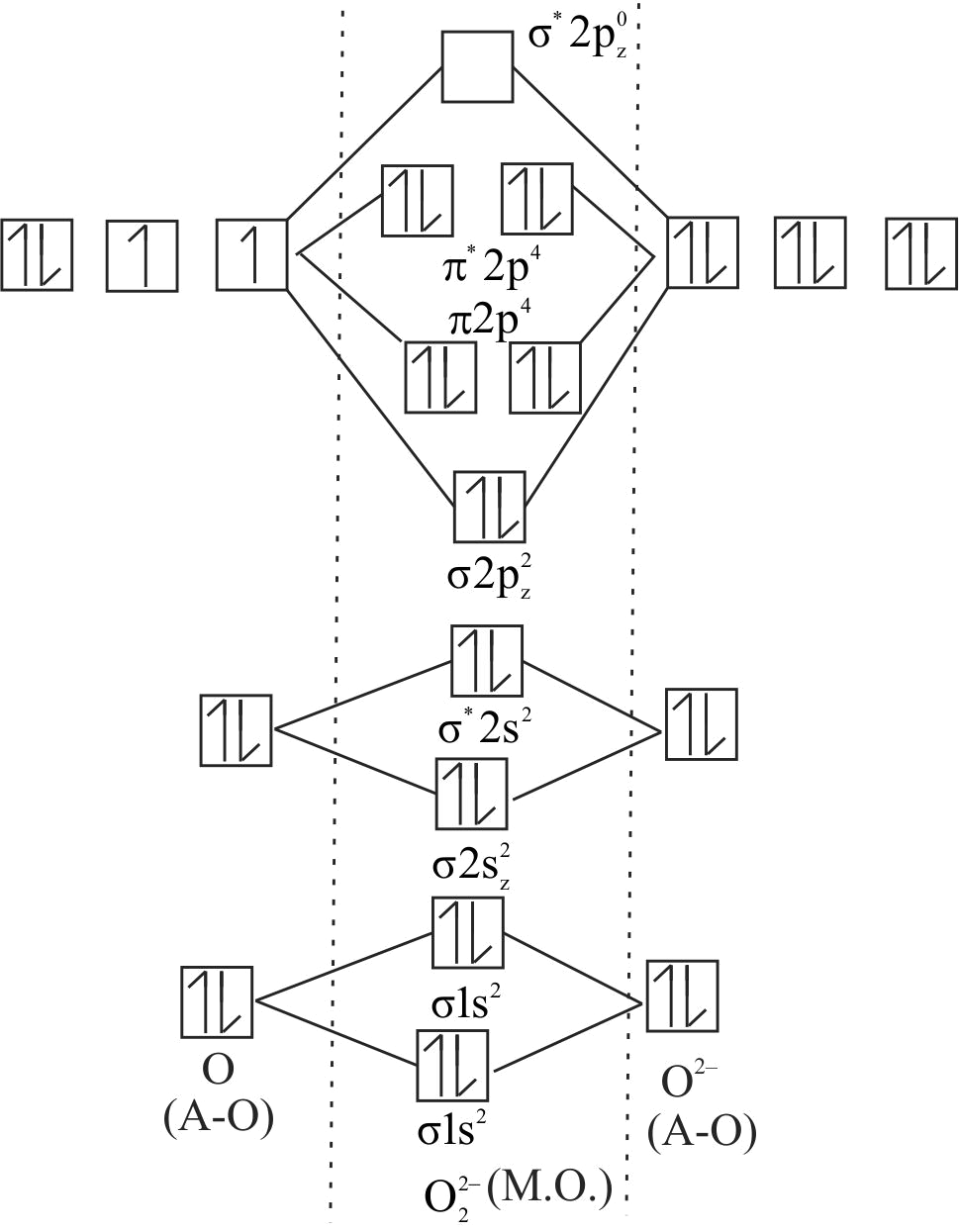
313714 Molecular orbital electronic configuration for ' \(\mathrm{X}\) ' anion is \(\mathrm{KK}\) \(\sigma^{*} 2 \mathrm{~s}^{2} \sigma^{*} 2 \mathrm{~s}^{2} \pi 2 \mathrm{p}_{\mathrm{x}}{ }^{2} 2 \mathrm{p}_{\mathrm{y}}{ }^{2} \sigma 2 \mathrm{p}_{\mathrm{z}}{ }^{2} \pi^{*} 2 \mathrm{p}_{\mathrm{x}}{ }^{1}\), the anion ' \(\mathrm{X}\) ' is
313714 Molecular orbital electronic configuration for ' \(\mathrm{X}\) ' anion is \(\mathrm{KK}\) \(\sigma^{*} 2 \mathrm{~s}^{2} \sigma^{*} 2 \mathrm{~s}^{2} \pi 2 \mathrm{p}_{\mathrm{x}}{ }^{2} 2 \mathrm{p}_{\mathrm{y}}{ }^{2} \sigma 2 \mathrm{p}_{\mathrm{z}}{ }^{2} \pi^{*} 2 \mathrm{p}_{\mathrm{x}}{ }^{1}\), the anion ' \(\mathrm{X}\) ' is
313714 Molecular orbital electronic configuration for ' \(\mathrm{X}\) ' anion is \(\mathrm{KK}\) \(\sigma^{*} 2 \mathrm{~s}^{2} \sigma^{*} 2 \mathrm{~s}^{2} \pi 2 \mathrm{p}_{\mathrm{x}}{ }^{2} 2 \mathrm{p}_{\mathrm{y}}{ }^{2} \sigma 2 \mathrm{p}_{\mathrm{z}}{ }^{2} \pi^{*} 2 \mathrm{p}_{\mathrm{x}}{ }^{1}\), the anion ' \(\mathrm{X}\) ' is
313714 Molecular orbital electronic configuration for ' \(\mathrm{X}\) ' anion is \(\mathrm{KK}\) \(\sigma^{*} 2 \mathrm{~s}^{2} \sigma^{*} 2 \mathrm{~s}^{2} \pi 2 \mathrm{p}_{\mathrm{x}}{ }^{2} 2 \mathrm{p}_{\mathrm{y}}{ }^{2} \sigma 2 \mathrm{p}_{\mathrm{z}}{ }^{2} \pi^{*} 2 \mathrm{p}_{\mathrm{x}}{ }^{1}\), the anion ' \(\mathrm{X}\) ' is