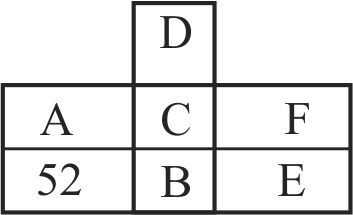
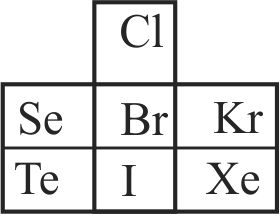313188 The number of \(\mathrm{Cl}\) atoms ionised in the process \(\mathrm{Cl} \rightarrow \mathrm{Cl}^{+}+\mathrm{e}\) by the energy liberated for the process \(\mathrm{Cl}+\mathrm{e} \rightarrow \mathrm{Cl}^{-}\)for one Avogadro number of atoms is \({\rm{x}} \times {10^{23}}.\) Given \(\mathrm{IE}=\) \(13.0\,\,{\rm{eV}}\) and \({{\rm{E}}_{\rm{g}}} = 3.60\,\,{\rm{eV}}.\) The value of x is ____.
313188 The number of \(\mathrm{Cl}\) atoms ionised in the process \(\mathrm{Cl} \rightarrow \mathrm{Cl}^{+}+\mathrm{e}\) by the energy liberated for the process \(\mathrm{Cl}+\mathrm{e} \rightarrow \mathrm{Cl}^{-}\)for one Avogadro number of atoms is \({\rm{x}} \times {10^{23}}.\) Given \(\mathrm{IE}=\) \(13.0\,\,{\rm{eV}}\) and \({{\rm{E}}_{\rm{g}}} = 3.60\,\,{\rm{eV}}.\) The value of x is ____.
313188 The number of \(\mathrm{Cl}\) atoms ionised in the process \(\mathrm{Cl} \rightarrow \mathrm{Cl}^{+}+\mathrm{e}\) by the energy liberated for the process \(\mathrm{Cl}+\mathrm{e} \rightarrow \mathrm{Cl}^{-}\)for one Avogadro number of atoms is \({\rm{x}} \times {10^{23}}.\) Given \(\mathrm{IE}=\) \(13.0\,\,{\rm{eV}}\) and \({{\rm{E}}_{\rm{g}}} = 3.60\,\,{\rm{eV}}.\) The value of x is ____.
313188 The number of \(\mathrm{Cl}\) atoms ionised in the process \(\mathrm{Cl} \rightarrow \mathrm{Cl}^{+}+\mathrm{e}\) by the energy liberated for the process \(\mathrm{Cl}+\mathrm{e} \rightarrow \mathrm{Cl}^{-}\)for one Avogadro number of atoms is \({\rm{x}} \times {10^{23}}.\) Given \(\mathrm{IE}=\) \(13.0\,\,{\rm{eV}}\) and \({{\rm{E}}_{\rm{g}}} = 3.60\,\,{\rm{eV}}.\) The value of x is ____.