313068
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power " n " i.e. \(\mathrm{v}^{\mathrm{n}}\) of X-rays emitted is plotted against atomic number 'Z', following graph is obtained. The value of " n " is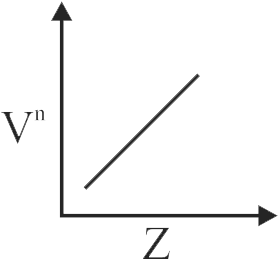
313068
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power " n " i.e. \(\mathrm{v}^{\mathrm{n}}\) of X-rays emitted is plotted against atomic number 'Z', following graph is obtained. The value of " n " is
313068
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power " n " i.e. \(\mathrm{v}^{\mathrm{n}}\) of X-rays emitted is plotted against atomic number 'Z', following graph is obtained. The value of " n " is
313068
It is observed that characteristic X-ray spectra of elements show regularity. When frequency to the power " n " i.e. \(\mathrm{v}^{\mathrm{n}}\) of X-rays emitted is plotted against atomic number 'Z', following graph is obtained. The value of " n " is