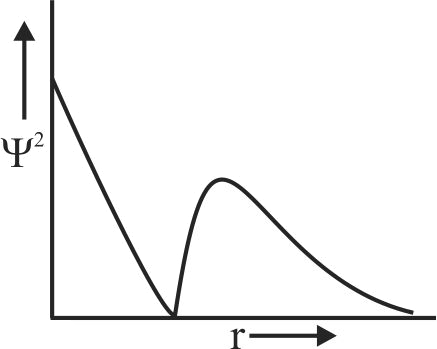
307532 The five orbitals are designated as \({{\rm{d}}_{{\rm{xy}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{yz}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{xz}}}}{\rm{,}}\,\,{{\rm{d}}_{{{\rm{x}}^{\rm{2}}}{\rm{ - }}{{\rm{y}}^{\rm{2}}}}}\) and \({{\rm{d}}_{{{\rm{z}}^{\rm{2}}}}}\). Choose the correct statement
307532 The five orbitals are designated as \({{\rm{d}}_{{\rm{xy}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{yz}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{xz}}}}{\rm{,}}\,\,{{\rm{d}}_{{{\rm{x}}^{\rm{2}}}{\rm{ - }}{{\rm{y}}^{\rm{2}}}}}\) and \({{\rm{d}}_{{{\rm{z}}^{\rm{2}}}}}\). Choose the correct statement
307532 The five orbitals are designated as \({{\rm{d}}_{{\rm{xy}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{yz}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{xz}}}}{\rm{,}}\,\,{{\rm{d}}_{{{\rm{x}}^{\rm{2}}}{\rm{ - }}{{\rm{y}}^{\rm{2}}}}}\) and \({{\rm{d}}_{{{\rm{z}}^{\rm{2}}}}}\). Choose the correct statement
307532 The five orbitals are designated as \({{\rm{d}}_{{\rm{xy}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{yz}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{xz}}}}{\rm{,}}\,\,{{\rm{d}}_{{{\rm{x}}^{\rm{2}}}{\rm{ - }}{{\rm{y}}^{\rm{2}}}}}\) and \({{\rm{d}}_{{{\rm{z}}^{\rm{2}}}}}\). Choose the correct statement
307532 The five orbitals are designated as \({{\rm{d}}_{{\rm{xy}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{yz}}}}{\rm{,}}\,\,{{\rm{d}}_{{\rm{xz}}}}{\rm{,}}\,\,{{\rm{d}}_{{{\rm{x}}^{\rm{2}}}{\rm{ - }}{{\rm{y}}^{\rm{2}}}}}\) and \({{\rm{d}}_{{{\rm{z}}^{\rm{2}}}}}\). Choose the correct statement
.png)