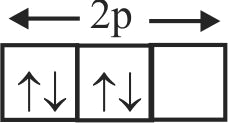
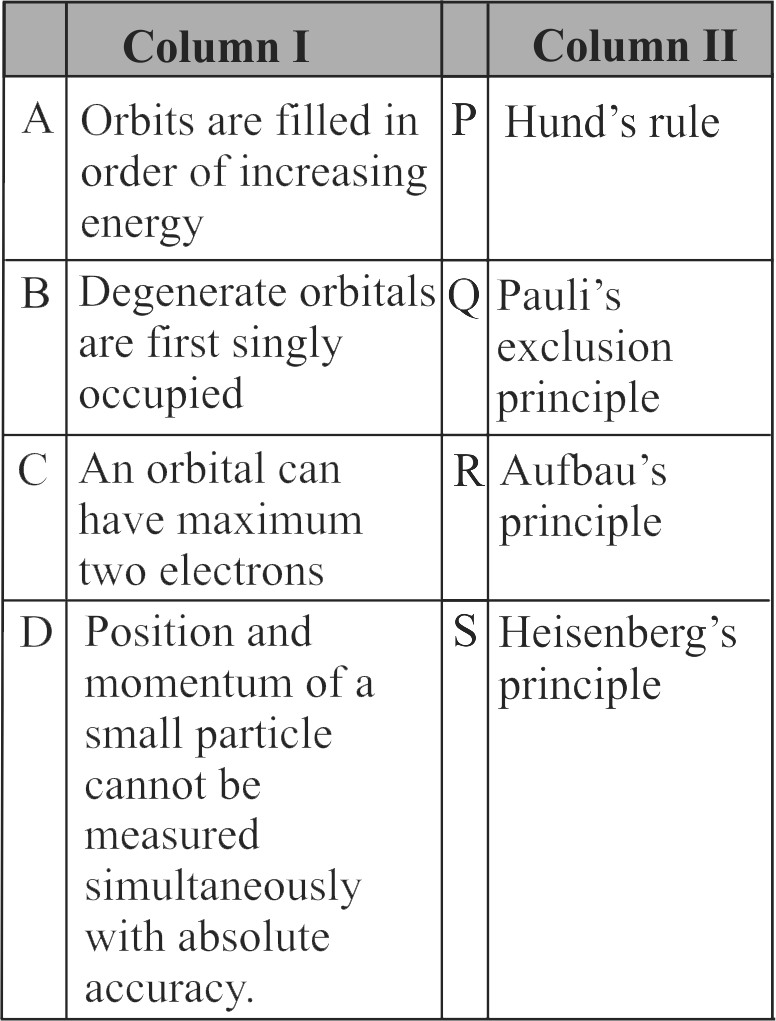
307337
The elctrons identified by quantum numbers n and l
\({\rm{(1)}}\,\,{\rm{n = 4,l = 1}}\,\,\,\,\,\,{\rm{(2)}}{\mkern 1mu} \,{\rm{n = 4,l = 0}}\)
\({\rm{(3)}}\,\,{\rm{n = 3,}}\,{\rm{l = 2}}\,\,\,\,\,{\rm{(4)}}\,\,{\rm{n = 3,}}\,{\rm{l = 1}}\)
can be placed in the order of increasing energy as
307337
The elctrons identified by quantum numbers n and l
\({\rm{(1)}}\,\,{\rm{n = 4,l = 1}}\,\,\,\,\,\,{\rm{(2)}}{\mkern 1mu} \,{\rm{n = 4,l = 0}}\)
\({\rm{(3)}}\,\,{\rm{n = 3,}}\,{\rm{l = 2}}\,\,\,\,\,{\rm{(4)}}\,\,{\rm{n = 3,}}\,{\rm{l = 1}}\)
can be placed in the order of increasing energy as
307337
The elctrons identified by quantum numbers n and l
\({\rm{(1)}}\,\,{\rm{n = 4,l = 1}}\,\,\,\,\,\,{\rm{(2)}}{\mkern 1mu} \,{\rm{n = 4,l = 0}}\)
\({\rm{(3)}}\,\,{\rm{n = 3,}}\,{\rm{l = 2}}\,\,\,\,\,{\rm{(4)}}\,\,{\rm{n = 3,}}\,{\rm{l = 1}}\)
can be placed in the order of increasing energy as
307337
The elctrons identified by quantum numbers n and l
\({\rm{(1)}}\,\,{\rm{n = 4,l = 1}}\,\,\,\,\,\,{\rm{(2)}}{\mkern 1mu} \,{\rm{n = 4,l = 0}}\)
\({\rm{(3)}}\,\,{\rm{n = 3,}}\,{\rm{l = 2}}\,\,\,\,\,{\rm{(4)}}\,\,{\rm{n = 3,}}\,{\rm{l = 1}}\)
can be placed in the order of increasing energy as
307337
The elctrons identified by quantum numbers n and l
\({\rm{(1)}}\,\,{\rm{n = 4,l = 1}}\,\,\,\,\,\,{\rm{(2)}}{\mkern 1mu} \,{\rm{n = 4,l = 0}}\)
\({\rm{(3)}}\,\,{\rm{n = 3,}}\,{\rm{l = 2}}\,\,\,\,\,{\rm{(4)}}\,\,{\rm{n = 3,}}\,{\rm{l = 1}}\)
can be placed in the order of increasing energy as
_.png)