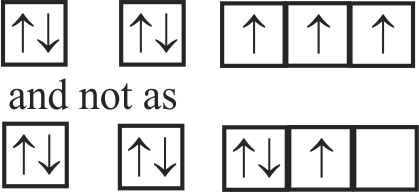
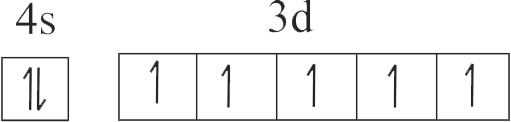
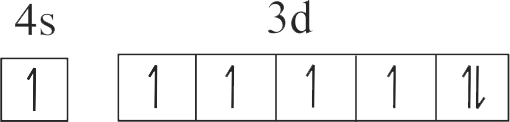
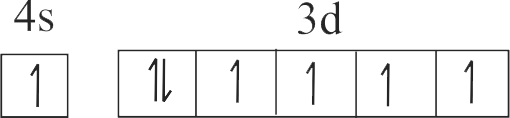
307269
Match the column I (element or ion) with column II (It’s electronic configuration) and choose the correct option.
| Column I | Column II |
|---|---|
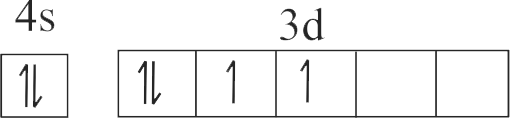
| A. \({\rm{Cu}}\) | P. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}\) |
| B. \({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}\) | Q. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{3}}}\) |
| C. \({\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}\) | R. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\) |
| D. \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}\) | S. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{9}}}\) |
307269
Match the column I (element or ion) with column II (It’s electronic configuration) and choose the correct option.
| Column I | Column II |
|---|---|
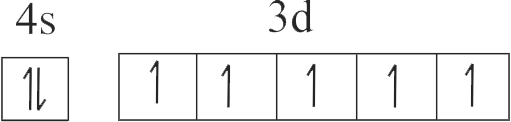
| A. \({\rm{Cu}}\) | P. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}\) |
| B. \({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}\) | Q. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{3}}}\) |
| C. \({\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}\) | R. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\) |
| D. \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}\) | S. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{9}}}\) |
307269
Match the column I (element or ion) with column II (It’s electronic configuration) and choose the correct option.
| Column I | Column II |
|---|---|
| A. \({\rm{Cu}}\) | P. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}\) |
| B. \({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}\) | Q. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{3}}}\) |
| C. \({\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}\) | R. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\) |
| D. \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}\) | S. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{9}}}\) |
307269
Match the column I (element or ion) with column II (It’s electronic configuration) and choose the correct option.
| Column I | Column II |
|---|---|
| A. \({\rm{Cu}}\) | P. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}\) |
| B. \({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}\) | Q. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{3}}}\) |
| C. \({\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}\) | R. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\) |
| D. \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}\) | S. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{9}}}\) |
307269
Match the column I (element or ion) with column II (It’s electronic configuration) and choose the correct option.
| Column I | Column II |
|---|---|
| A. \({\rm{Cu}}\) | P. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}\) |
| B. \({\rm{C}}{{\rm{u}}^{{\rm{2 + }}}}\) | Q. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{3}}}\) |
| C. \({\rm{Z}}{{\rm{n}}^{{\rm{2 + }}}}\) | R. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{{\rm{10}}}}{\rm{4}}{{\rm{s}}^{\rm{1}}}\) |
| D. \({\rm{C}}{{\rm{r}}^{{\rm{3 + }}}}\) | S. \({\rm{1}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{s}}^{\rm{2}}}{\rm{2}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{s}}^{\rm{2}}}{\rm{3}}{{\rm{p}}^{\rm{6}}}{\rm{3}}{{\rm{d}}^{\rm{9}}}\) |