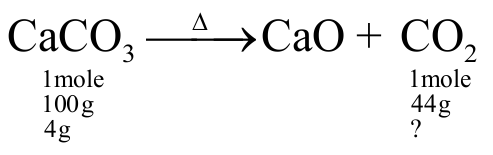307048
The right option for the mass of \(\mathrm{CO}_{2}\) produced by heating \(20 \mathrm{~g}\) of \(20 \%\) pure limestone is (Atomic mass of \(\mathrm{Ca}=40\) )
\(\left[ {{\text{CaC}}{{\text{O}}_{\text{3}}}\xrightarrow{{{\text{1200 K}}}}{\text{CaO + C}}{{\text{O}}_{\text{2}}}} \right]\)
307048
The right option for the mass of \(\mathrm{CO}_{2}\) produced by heating \(20 \mathrm{~g}\) of \(20 \%\) pure limestone is (Atomic mass of \(\mathrm{Ca}=40\) )
\(\left[ {{\text{CaC}}{{\text{O}}_{\text{3}}}\xrightarrow{{{\text{1200 K}}}}{\text{CaO + C}}{{\text{O}}_{\text{2}}}} \right]\)
307048
The right option for the mass of \(\mathrm{CO}_{2}\) produced by heating \(20 \mathrm{~g}\) of \(20 \%\) pure limestone is (Atomic mass of \(\mathrm{Ca}=40\) )
\(\left[ {{\text{CaC}}{{\text{O}}_{\text{3}}}\xrightarrow{{{\text{1200 K}}}}{\text{CaO + C}}{{\text{O}}_{\text{2}}}} \right]\)
307048
The right option for the mass of \(\mathrm{CO}_{2}\) produced by heating \(20 \mathrm{~g}\) of \(20 \%\) pure limestone is (Atomic mass of \(\mathrm{Ca}=40\) )
\(\left[ {{\text{CaC}}{{\text{O}}_{\text{3}}}\xrightarrow{{{\text{1200 K}}}}{\text{CaO + C}}{{\text{O}}_{\text{2}}}} \right]\)