306642
Statement A :
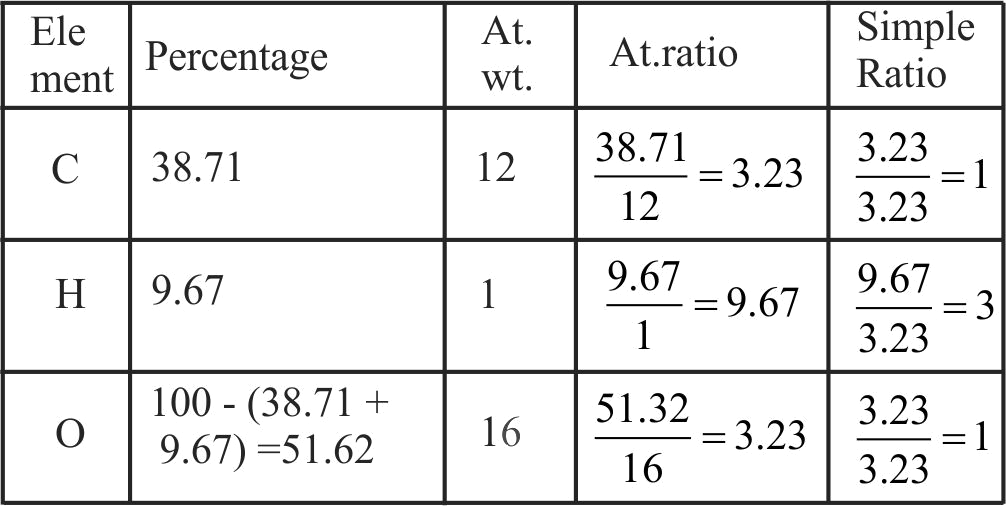
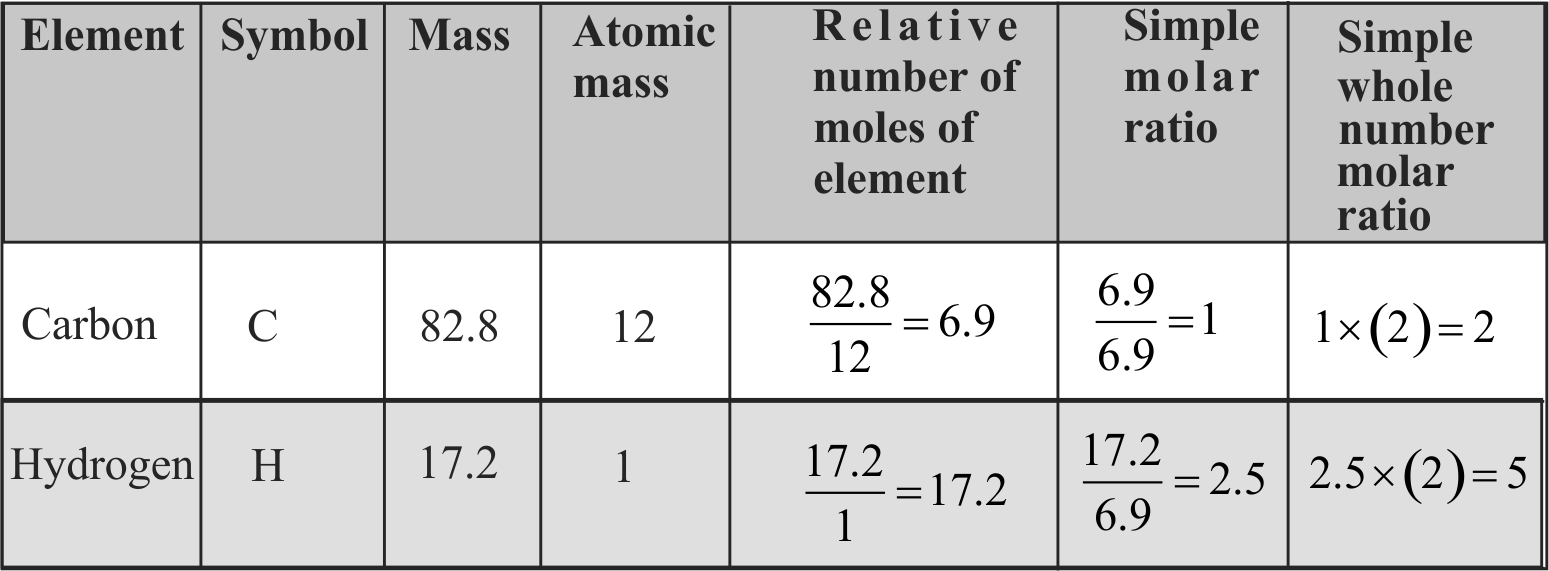
Molecular formula shows the exact number of different types of atoms present in a compound.
Statement B :
Molecular formula can be obtained directly from empirical formula which represents the simplest whole number ratio of various atoms present in a compound.
306642
Statement A :
Molecular formula shows the exact number of different types of atoms present in a compound.
Statement B :
Molecular formula can be obtained directly from empirical formula which represents the simplest whole number ratio of various atoms present in a compound.
306642
Statement A :
Molecular formula shows the exact number of different types of atoms present in a compound.
Statement B :
Molecular formula can be obtained directly from empirical formula which represents the simplest whole number ratio of various atoms present in a compound.
306642
Statement A :
Molecular formula shows the exact number of different types of atoms present in a compound.
Statement B :
Molecular formula can be obtained directly from empirical formula which represents the simplest whole number ratio of various atoms present in a compound.