1 $-\mathrm{NH}_{2}$ group is highly metro-directive
2 $-\mathrm{NO}_{2}$ substation always takes place at metroposition
3 Formation of anilinium ion
4 Low temperature
Explanation:
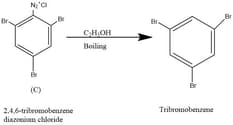
When diazonium compounds are heated with ethanol, the diazonium ion is replaced by a hydrogen atom and benzene would be formed. Thus, Z is 2,4,6 tribromobenzene diazonium chloride.
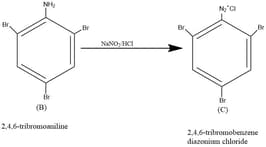
Aromatic amines on heating with \(NaNO_{2}/HCl\)
gives diazonium compounds. Hence, Y is 2,4,6 tribromoaniline.
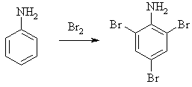
Aniline on bromination gives 2,4,6 tribromoaniline. Hence, X is aniline.