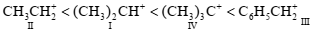231621
Consider the following carbocations
I. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}^{+}\)
II. \(\mathbf{C H}_3 \mathbf{C H}_2^{+}\)
III. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2^{+}\)
IV. \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+}\)
The correct sequence for the stability of these is
231621
Consider the following carbocations
I. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}^{+}\)
II. \(\mathbf{C H}_3 \mathbf{C H}_2^{+}\)
III. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2^{+}\)
IV. \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+}\)
The correct sequence for the stability of these is
231621
Consider the following carbocations
I. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}^{+}\)
II. \(\mathbf{C H}_3 \mathbf{C H}_2^{+}\)
III. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2^{+}\)
IV. \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+}\)
The correct sequence for the stability of these is
231621
Consider the following carbocations
I. \(\left(\mathrm{CH}_3\right)_2 \mathrm{CH}^{+}\)
II. \(\mathbf{C H}_3 \mathbf{C H}_2^{+}\)
III. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2^{+}\)
IV. \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}^{+}\)
The correct sequence for the stability of these is